Authors: Marco Gambacciani and Marco Levancini
Published in: The Journal of The North American Menopause Society. 2017;24(3):316-319
1. A MINIMALLY INVASIVE SOLUTION FOR BREAST CANCER SURVIVORS
The objective of the study was to evaluate the efficacy and acceptability of the RenovaLase® procedure for treating patients with premature GSM due to estrogen blocking therapy.
2. METHODOLOGY
Forty-three postmenopausal breast cancer survivors received 3 RenovaLase® treatments with 30 days in-between the sessions. Symptoms were evaluated before the treatment and after 1, 3, 6, 12, and 18 months using two methods: subjective Visual Analog Scale (VAS) and objective Vaginal Health Index Score (VHIS).
3. VERY PROMISING RESULTS
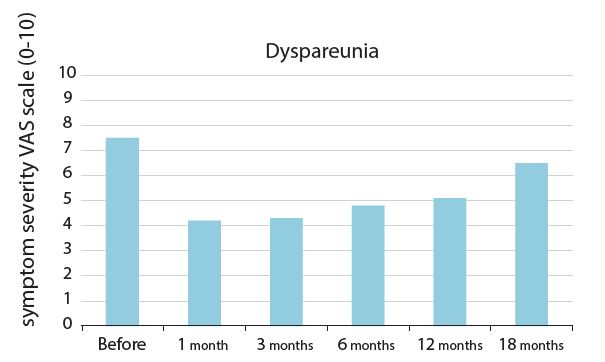
VAS values for vaginal dryness showed a statistically significant reduction from baseline 8.5±1.0 cm to 4.4±1.2 cm after 3 months, to 5.5±1.5 cm after 12 months, and returned to nearly baseline levels at 18 months (NS vs basal values). VAS values for dyspareunia followed a similar pattern. VHIS score showed a statistically significant increase from baseline values of 8.1±1.3 to 21.0±1.4 after the third treatment and to 18±1.8 at twelve months from the final laser treatment. VHIS score was kept above baseline values even after 18 months from the final treatment (NS vs basal values).
4. RENOVALASE IS A SAFE TREATMENT FOR BREAST CANCER SURVIVORS
Results from this study indicate that RenovaLase® is a treatment option for GSM in breast cancer patients whose current treatment options are still very limited.